The first recognized adenoviral infections of birds were quail bronchitis and chicken embryo lethal
orphan (CELO) viruses, which were known in 1949 and 1957, respectively. Later, inclusion bodies in
chicken livers were described in 1963, followed by the isolation of a “new agent” of a disease called
“inclusion body hepatitis (IBH)” in 1973. However, for many years the exact role of adenoviruses in
causing avian diseases was unclear. Adenoviruses are suspected of playing a secondary role in causing
many syndromes. For instance, the presence of immunosuppressive viruses, such as chicken anemia
virus (CAV) or infectious bursal disease virus (IBDV) has been reported to enhance the pathogenicity of
some adenoviruses to cause IBH. However, there is evidence that adenoviruses cause IBH without a
requirement for other pathogens. Today, IBH has a worldwide distribution, affecting domestic species of
all ages, and with indication that the incidence of the disease is increasing.
The adenoviruses are members of the family Adenoviridae, which is divided in four genera namely
Mastadenovirus infecting mammals, and Aviadenovirus, Siadenovirus, and Atadenovirus infecting
birds. The latter three genera are classified as Group I, II and III avian adenoviruses respectively.
Adenoviruses are icosahedral, non-enveloped double stranded DNA viruses that range in size from 70
to 100 nm and have 252 capsomeres surrounding a core. Adenoviruses replicate in the nucleus,
producing characteristic inclusion bodies. With respect to several characteristics of the virion, such as
virus morphology or genome organization, which are relevant for diagnostic purposes, the avian
adenoviruses are very heterogeneous. Consequently, the diagnosis of avian adenoviruses differs
significantly among the three different groups.
At least 12 serotypes of fowl Aviadenovirus have been recognized on the basis of virus neutralization
tests (with several strains in each serotype). These serotypes and the other aviadenoviruses share a
common group antigen. Under a new classification scheme, which considers additional criteria, such as
calculated phylogenetic distance and restriction fragment length polymorphism analysis of the genome,
the 12 serotypes were assigned to one of five virus species i.e., Fowl adenovirus (FAdV) A-E. Only
serotype 1 (Fowl adenovirus A or FAdV-A) has haemagglutination activity, but it agglutinates only rat red
blood cells. Exposure to one serotype confers no immunity to other serotypes within the group I (NO
CROSS PROTECTIVE IMMUNITY AMONG SEROTYPES FOUND). Similarly, infections with a strain of the
group I will not protect against infections with viruses from the group II or III. For these reasons it is not
uncommon to isolate two serotypes from the same bird and a broiler flock may have four or more
serotypes present. There is also little protection between the 12 serotypes of aviadenoviruses.
Considerable exchange of serotypes may occur when commercial flocks are made up of the progeny of
several parent flocks. At sexual maturity a bird may have been infected with the majority of the 12
recognized serotypes.
Following experimental infection of specific-pathogen-free (SPF) chicks in the first days of life, using
natural routes of exposure, initial growth of fowl adenoviruses occurs mainly in the intestinal
epithelium, followed by a viraemia, and the presence of the virus in many organs (liver, kidney,
respiratory tract, bursa of Fabricius, spleen and bone marrow). However, in the field, infections with
aviadenovirus are not normally detected during the first few days of life although isolations from 3
weeks onwards are common. In naturally occurring infections, aviadenovirus is excreted in the feces for
about 3 weeks, with the peak excretion occurring between 4 to 7 days after infection. Certainly, birds
can excrete one serotype in spite of high levels of neutralizing antibody to other serotypes.
Although aviadenoviruses have been isolated from a number of clinical conditions, there is no clear
evidence for a primary role in disease causation. However, aviadenoviruses have been most commonly
associated with IBH (mainly types D and E), hydropericardium syndrome (type C), gizzard erosions (type
A), and respiratory diseases. Aviadenoviruses have also been suspected as a cause of egg production

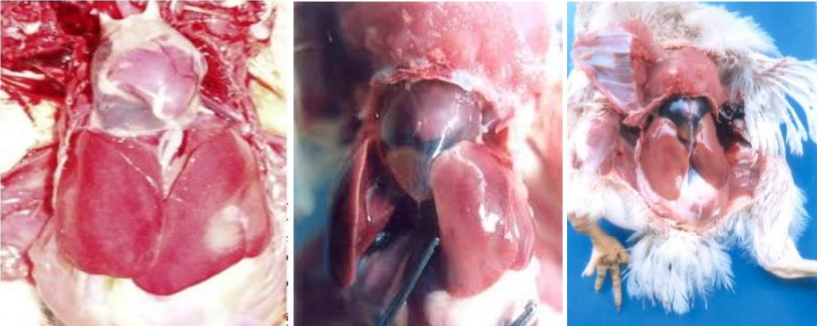
Image-1: IBH. Birds showed lethargy, huddling with ruffled
feathers and lack of appetite
problems in laying hens
(EDS) and
viral arthritis/tenosynovitis.
Inclusion body hepatitis of chickens was first described in the U.S. in 1963. Since then, the disease has
been reported worldwide. A sharp rise in severity and occurrence of IBH has been reported. This disease
is usually seen in 2 to 3 week-old broilers (sometimes as young as 4 days to 7 weeks of age). Other
species, such as pigeon, guinea-fowl, psittacines, or turkey may be affected. Naturally occurring
outbreaks have been associated with a wide spectrum of serotypes. Aviadenoviruses are primary
pathogens for IBH although co-infection with IBDV or CAV has been reported to increase
pathogenicity.
IBH is characterized by a sudden increase of mortality that generally peaks within 3 to 4 days and ceases
within 9 to 14 days. Mortality normally ranges from 2 to 10%. However, there have been outbreaks in
which mortality has reached 30% depending of the pathogenicity of the virus, immune status of the
affected birds, and concurrent secondary infections. Clinically, the birds show lethargy, huddling with
ruffled feathers, stooping, inappetence, and yellow and mucoid droppings may be seen. Overall feed
conversion and weight gain are usually depressed.
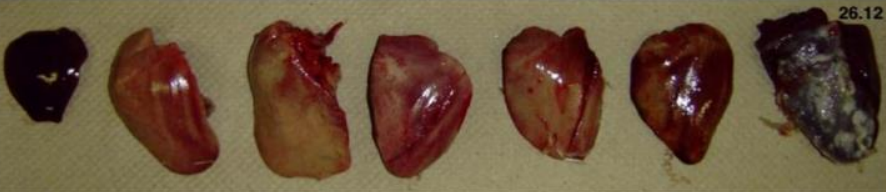
Gross lesions in dead birds include an enlarged, pale, and friable liver sometimes with necrotic foci.
Hemorrhages are frequently seen in the liver and sometimes in leg and breast muscles (which usually
considered as sign of Gumboro). The kidneys are enlarged, pale, and mottled with multiple
hemorrhages. In some cases, hydropericardium can be observed. Necrotizing pancreatitis and
intranuclear inclusion bodies have also been reported in some outbreaks,
particularly in guinea fowl. In addition, enlarged spleens and thymus atrophy can be seen in most dead
birds. Anemia, icterus of the skin and subcutaneous fat, hemorrhages in various organs, and bone
marrow degeneration are usually present, but vary in severity. In some cases, gizzard erosions can be
seen. Microscopically, there are necrotic focal lesions in the gizzard. In the liver, eosinophilic (or
basophilic) inclusion bodies are present in the hepatocytes.
This condition was first recognized from the village of Angara near Karachi in Pakistan in 1987 hence
named “Angara” disease. The disease is similar to IBH with higher mortality ranging from 20 to 80% in
broilers. Hydropericardium syndrome is characterized by the accumulation of up to 10 ml of fluid in the
pericardium. Aviadenoviruses belonging mostly to serotype 4 have been implicated in the causation of
this condition. Hydropericardium syndrome affects mainly broiler chicks 3 to 6 weeks of age and is
caused by FAdV-4. With a course of 7 to 15 days, Hydropericardium syndrome is mainly characterized by
rapidly increasing mortality. During the last stages of disease, affected birds exhibit dullness,
depression, ruffled feathers, huddling, ventral recumbency, and closed eyes.
Fluid in the pericardial sac may accumulate due to transudate, inflammatory process in the pericardium,
shunting of blood from the ventricles or large vessels into the pericardial cavity. Pathological fluid in the
pericardial sac does not cause major hemodynamic disorders until the intra pericardial pressure is
normal. This condition is treated mainly pharmacologically. Severe tamponade may result in cardiac
arrest due to electromechanical dissociation. Cardiac tamponade may be due to all causes of fluid
accumulation in the pericardial sac, but most frequently it results from perforation or rupture of the left
ventricle or aorta, and due to severe idiopathic/viral, uremic or neoplastic pericarditis. Treatment of
pericarditis and reduction of inflammatory process in pericardium may reduce death rates due to
pericardial fluids.
Following infection, birds rapidly developed neutralizing (type-specific) antibodies that were detectable
after 1 week and reached peak titers after 3 weeks. It has been found that birds were resistant to
reinfection with the same serotype 45 days after primary infection, whereas birds were successfully
reinfected with the same strain after 8 weeks, eliciting a secondary response of both neutralizing and
precipitating antibodies.
Virus excretion also occurred, despite the presence of humoral antibodies. Peaks of virus excretion were
found when birds were 2.5, 4.5, and 7.5 months of age, consistent with the theory that local immunity
lasts about 8 weeks. Therefore, it is possible that the resistance to challenge found after infection is due
to short-lived local immunity while circulating antibodies protect mainly against invasion of the internal
organs. The apparent correlation between appearance of circulating antibodies and cessation of virus
excretion is more likely due to concurrent development of both local immunity, which is more transient,
and humoral immunity, which is more persistent. Support for this hypothesis is provided by the finding
that maternal antibody do not protect against natural routes of infection but does protect against intraabdominal infection.
Importance of immune enhancement theory against adenovirus: With regard to protection,
neutralizing antibodies are obviously not solely responsible for protection, determined in a severe
challenge model.
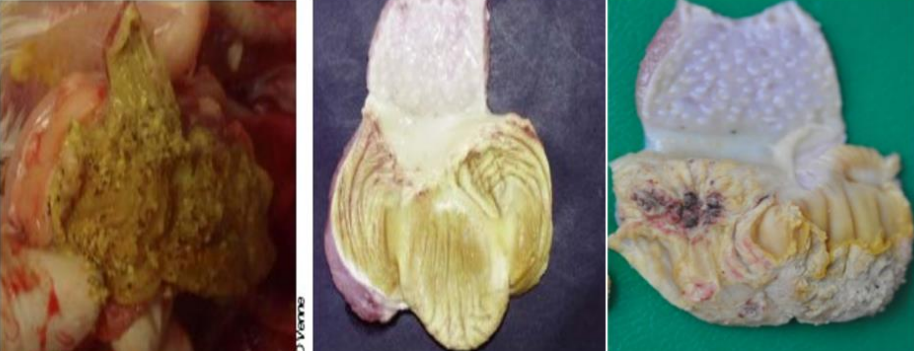
The main lesions of IBH are pale, friable, swollen livers. Small white foci can be seen on the liver, and
petechial or ecchymotic hemorrhages may be present in the liver and skeletal muscles. Inclusion bodies
are seen in the hepatocytes. These can be eosinophilic, large, round, or irregularly shaped with a clear
pale halo or occasionally basophilic. Virus particles were detected only in cells with basophilic inclusions,
and the eosinophilic inclusions were composed of a fibrillar, granular material. Lesions including atrophy
of the cloacal bursa and thymus, aplastic bone marrow, and hepatitis were described in natural
outbreaks and experimental studies. Glomerulonephritis determined by histomorphometric evaluation
was noticed during an outbreak of IBH.
Portal Developed and Maintained by Trèsbien Biosynth Pvt Ltd.
 9883 078810 | 7003 218193
9883 078810 | 7003 218193
 admin@tresbienbiosynth.com
admin@tresbienbiosynth.com
 info@tresbienbiosynth.com
info@tresbienbiosynth.com
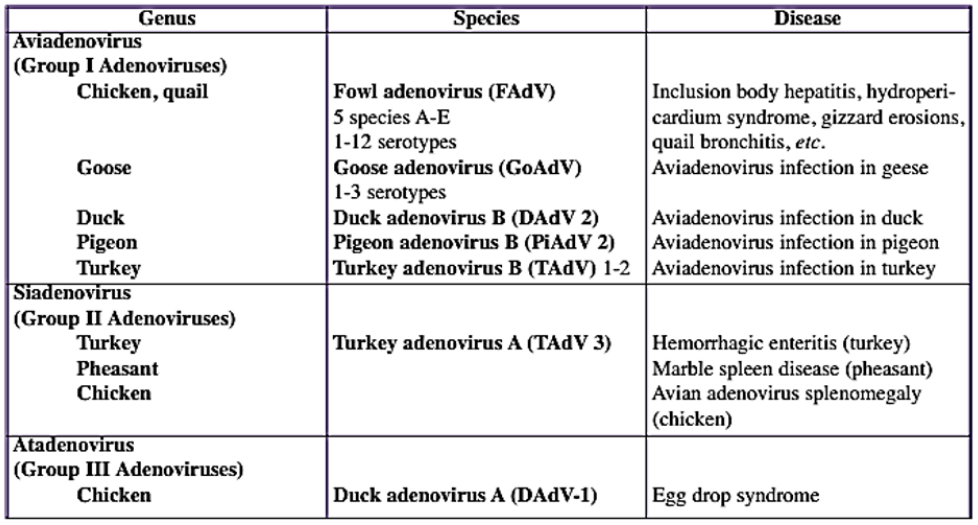

 Image-1: IBH. Birds showed lethargy, huddling with ruffled
feathers and lack of appetite
Image-1: IBH. Birds showed lethargy, huddling with ruffled
feathers and lack of appetite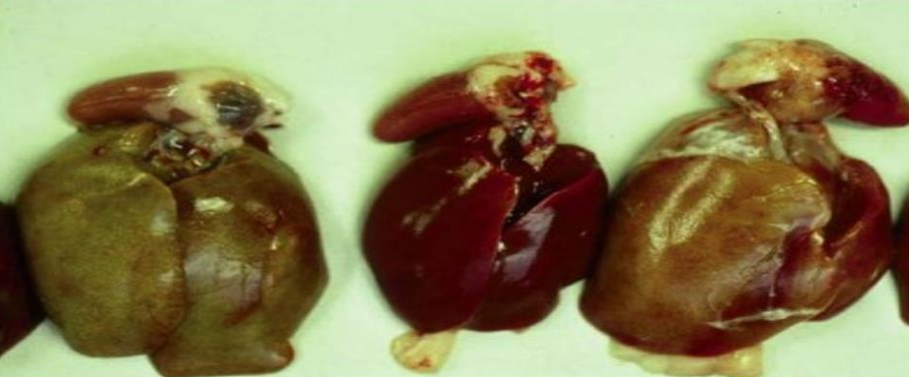 Image-1: IBH. Birds showed lethargy, huddling with ruffled
feathers and lack of appetite
Image-1: IBH. Birds showed lethargy, huddling with ruffled
feathers and lack of appetite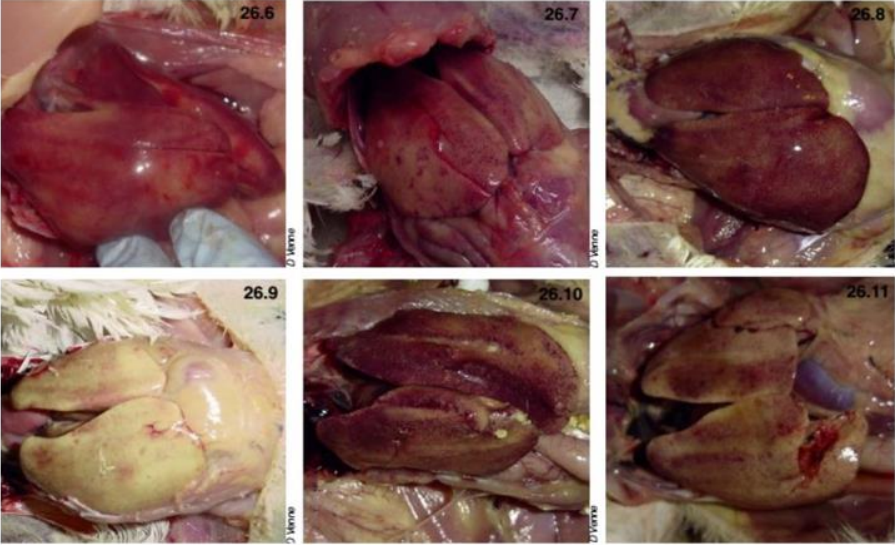
 9883 078810 | 7003 218193
9883 078810 | 7003 218193  admin@tresbienbiosynth.com
admin@tresbienbiosynth.com  info@tresbienbiosynth.com
info@tresbienbiosynth.com